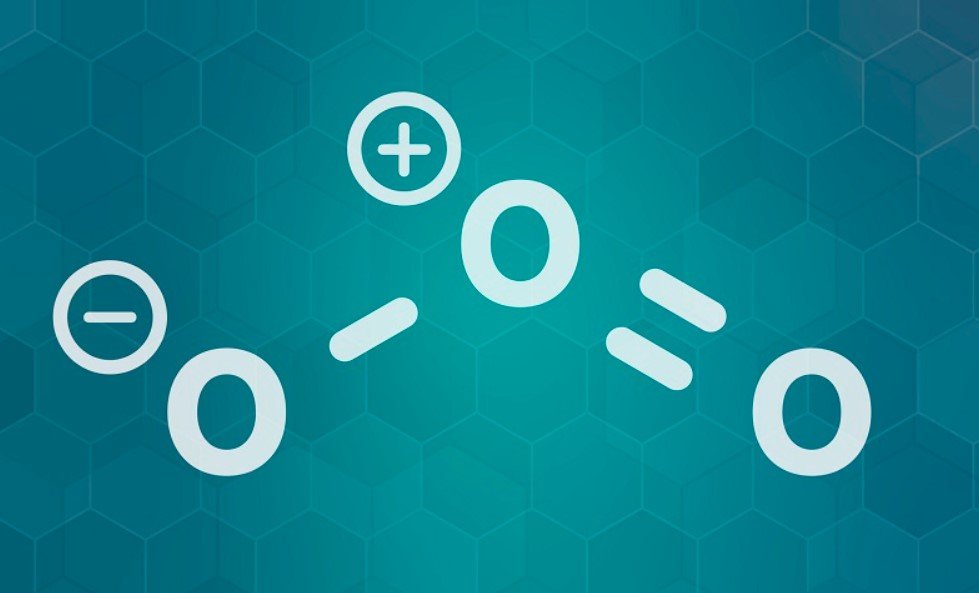
It is important to know what ozone is before understanding the ozone oxidation. With a formula of O3, ozone is a substance of oxygen. As a gas, ozone is colorless or light blue when it is at room temperature and pressure. It has a foul odor. A very unstable gas, ozone reacts and disappears very quickly. Our atmosphere contains ozone, which is a vital gas that protects us from harmful UV rays. The stratosphere, also known as the ozone layer, contains the highest concentration of ozone. In addition to being a greenhouse gas, ground-level ozone has a high oxidation potential. When certain air pollutants react with UV light (sunlight), ground-level ozone is produced. This reaction is called photolysis. This article covers all the information about ozone oxidation and how it can help in disinfection of water and air.
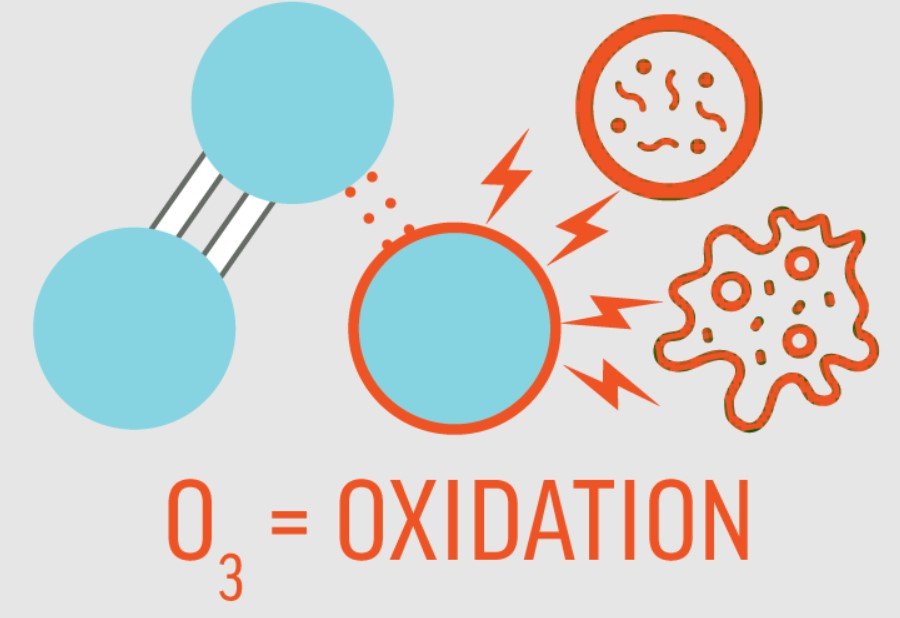
What is Oxidation?
Ozone is a powerful oxidizer, unlike normal oxygen. An oxidation reaction occurs when atoms, molecules, or ions give up electrons. A reduction reaction occurs when electrons are gained. It is not necessary for oxygen atoms or molecules to be involved in oxidation reactions. Most people who do not understand chemistry or who have forgotten high school chemistry think of oxidation as burning or rusting. The oxidation-reduction process is simply oxidation at the atomic level. In addition to liquids and gases, it occurs in solids.
Among the most powerful oxidants, ozone is only surpassed by fluorine, oxygen, and the hydroxyl radical. Ozone can create even higher oxidation potentials when combined with hydroxyl radicals in several reactions.
Oxygen’s high oxidation potential makes it capable of oxidizing many compounds not readily oxidized by other chemicals. Inorganic species such as FE+2 and I- are especially affected by this potential. Most often, though, there is no explicit electron transfer from an ozone molecule to another compound, but rather oxygen transfer.
In oxidation, a substance (reduction) transfers electrons to another substance (oxidizer), resulting in an increase in oxidation number. OH radicals react with ozone in water. In addition to oxidizing even stronger than ozone itself, these radicals are very short-lived.
How is Ozone Produced?

Ozone (O3) is created by converting oxygen (O2) from the air to high electrical voltage (Corona discharge).
How does Ozone Oxidation Work?
Oxidation by ozone is known to have the intended effect, so in fact it isn’t really a removal process since the components are not removed. Indirect reactions with OH Radicals or reactions with ozone are two ways to oxidize. Fresh water oxidizes certain components. When micro-genes are removed from a cell, their DNA and cell walls will be oxidized.
What are the Benefits of Ozone Oxidation?
- No transport required, because it is produced on location
- Widely applicable for oxidation of various components.
- Oxidized substances are easy to remove.
- There is no better way to disinfect and cleanse water than by ozone oxidation.
Must check out: SPA & Home Water Ozonizers
Ozone oxidation is by far the most effective water treatment process. Furthermore, ozone is an eco-friendly process since it breaks down into oxygen within minutes. This is due to the fact that ozone has a high oxidation potential, meaning it is capable of reacting with other substances. Using the redox potential as a measure of ozone’s oxidation potential, we find that ozone has about five times the oxidation potential of oxygen, and about twice that of chlorine. Viruses, bacteria, and fungus are all killed by ozone. Due to the high kill rate, reaction times can be shortened and reaction tanks can be smaller than with other oxidants. As a result, tank and treatment plant investment costs can be greatly reduced.
The ozone can not only kill microorganisms, but also control taste, odor, and color. Additionally, it is useful for flocculating organic materials and simplifying mechanical filtration.
How Ozone Oxidation Can Help You?
Moreover, ozone is often used to eliminate flavors, odors, and colors (organic components) besides pathogenic organisms, pathogens, bacteria, and viruses. Oxidizing ozone can also remove organic micro-contaminates, such as pesticides and medicines. Ozone purification techniques are more effective at removing oxidized substances since they are less soluble in water. A common application of ozone is in producing potable water, purifying swimming pool water and cooling water, controlling odors, and disinfecting waste water.
Some other common applications of ozone generator include:
- Medical treatment industry: operating room, medical treatment equipment, disinfect sickroom, aseptic room, etc.
- Waste/Sewerage water treatment (Agriculture wastewater treatment)
- Laboratory: industrial oxidation of flavor and pharmaceutical water treatment.
- Ozone laundry system for Washing machine
- Decolor for textile and Jeans bleaching
- Beverage industry: disinfect production water supply for Bottle water -pure water,
- mineral water and any kind of beverage, etc.
- Ozone use for surface sanitation
- Vegetables and fruits processing industry: keep vegetables and fruits fresh and Cold storage;
- disinfect production water supply for fruits and vegetables processing.
- Sea food: Remove smell of seafood factory and kill bacteria
- Poultry: Remove smell of poultry factory and kill bacteria, and disinfect water for poultry feeding.
- Swimming pool and SPA water sterilization and disinfection
- Aquarium and aquaculture water sterilization
You can go through detailed discussion on different ozone applications here.
You can let us know if you need help choosing the appropriate ozone generator for you. We will recommend the best options according to your needs. Just reach us out at our contact us page.




